Nuclear Fission: When science feels like magic
Nuclear fission bridges energy gaps: high-output, low-carbon, as reliable as fossil fuels with emissions rivaling renewables. This guide demystifies its science, balancing rigor & clarity to counter opposition fueled by misunderstanding.
Throughout history, only two moments have left me in profound awe, moments where I struggled to fully grasp the magnitude of our civilization’s progress. The first was discovering that we had cracked the genetic code and unlocked the very language of life. Deciphering the genetic code unlocked our ability to understand, diagnose, and treat genetic diseases; engineer life-saving medicines; tailor personalized medical treatments; and revolutionize fields like agriculture and biotechnology. This fundamentally transformed how we approach health, science, and life itself. My second awe-struck moment was learning we’d split the atom. Casually mentioned by my teacher, I was daydreaming its staggering implications, tuning out subsequent lecture. My daze grew so deep that I missed being addressed, until my friend nudged me. Rising sheepishly, I impulsively asked about breaking the atom rather than topic being discussed, earning baffled stares. That collision of wonder and embarrassment still remains vivid.
Later achievements paled in comparison, whether from adulthood’s jaded lens or the numbing rhythm of progress, nothing resonated like those twin leaps. Yet that awe curdled into dread when I grasped the cost. The very breakthrough that symbolized our mastery, the atom’s splitting, was first wielded not for enlightenment, but annihilation. Cities erased, lives vaporized. Twice. The brilliance of unlocking the universe’s secrets clashed, forever, with the shadow of what we chose to do with them.
To readers who may find the opening of this article unsettling, I offer my sincere apologies. Beginning a discussion on nuclear fission with the horrors of Hiroshima and Nagasaki is not a choice made lightly, its a refusal to let the scale of human loss fade into abstraction.
The atomic bombings of 1945 represent one of our humanity’s most sobering paradoxes: a scientific breakthrough capable of limitless energy was first weaponized to erase two cities, extinguishing between 110,000 - 210,000 lives. Families and communities vanished in a flash, with survivors left to endure radiation’s cruel aftermath. To gloss over this history would feel akin to silencing the voices of those who perished, reducing their stories to footnotes in a narrative of progress.
The same scientific principles that unleashed devastation in 1945 now power cities and sustain modern life. At its core, nuclear fission, begins with a neutron striking a heavy and unstable atom (like Uranium-235). This results in a highly unstable atom that splits instantly into into lighter fragments, releasing colossal energy and additional neutrons. These neutrons then bombard with other radioactive atoms to continue the chain reaction. In nuclear reactors, this chain reaction is carefully controlled and the energy heats water, spins turbines, and generates electricity.
Envisioning nuclear fission: my initial conceptual journey (click here)
As a child, I envisioned a scientist in a white lab coat, wearing micro-zoom goggles. Gripping the legendary Excalibur blade with both hands, he strained his eyes to spot an atom resting on a gleaming metallic surface. With a decisive swing, he split the atom in two, unleashing a thunderous burst of energy that was collected into two gigantic glass chambers.
It was when i grew up that i realized, instead of using the legendary blade to split the atom, we coerce a third party into the atom, effectively instigating a civil war inside the atom and spliting it into two different nations.. ahem., atoms and banishing the neutrons that didn't align with the newly formed atoms.
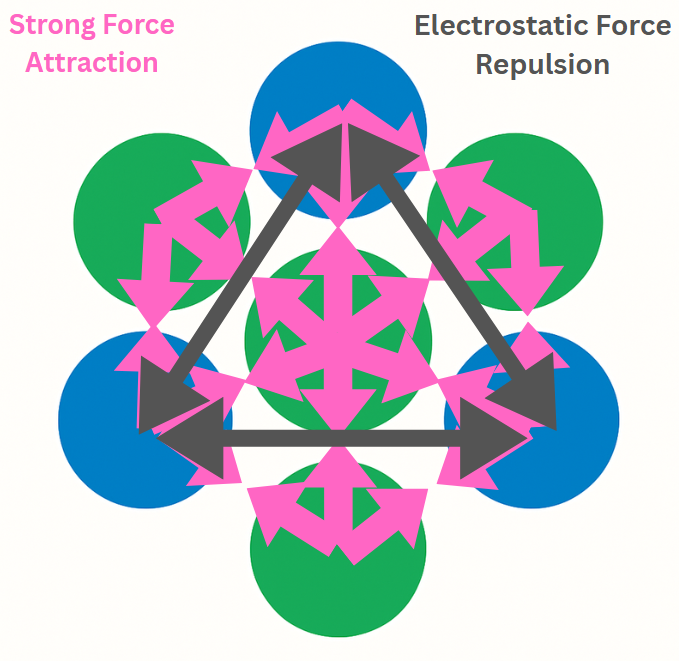
To figure out how break an atom, first we need to understand what makes the protons, neutrons and electrons stick together to form an atom. Protons and neutrons form the dense central nucleus of an atom, while electrons occupy surrounding regions called orbitals. Through oxidation–reduction (redox) reactions, valance electrons can be removed from or added to atoms, driving most chemical processes. Modifying protons and neutrons requires different kind of reactions, high‑energy processes that are significantly more complex and energetically demanding than electron transfer. For this we need to understand the two major forces acting at the nucleus.
- Strong force, which is said to be one of the strongest fundamental forces, acts only at sub-atomic distances pulling everything together regardless of their charge.
- Electrostatic force, which is weaker but can act from relatively longer distances. This causes like charges to repel each other and opposite charges to attract towards each other.
Mass defect and binding energy
Take any element, for instance Helium-4 with atomic weight of
4.0026032541 units
Helium 4 is a element with 2 protons, 2 neutrons and 2 electrons, we add the individual masses of protons, neutrons and electrons.
2x(1.007276) + 2x(1.008665) + 2x(0.00054858) units = 4.03297 units (4.03188 units, if we neglect the electrons)
Upon comparision, we can find the disparity in atomic mass of element and the summation of protons, neutrons and electrons that make up the element. The difference in these masses is called mass defect. We know from the theory of relativity, E = mc² , where E stands for energy and c represents the speed of light which is approximately 299,792,458 meters per second. The m denotes the mass and any mass multiplied by the speed of light gives a large amount of energy, so all matter is just extremely dense pool of energy.
In our case with the Helium 4, the mass defect multiplied by the speed of light (c), will give us the amount of energy we need to break the atom. This energy or work required to break the atom or a nucleus into its constituents is called binding energy. Since the masses are very small and it’s a hassle to convert the masses into kilograms, we directly use relative atomic mass units (u) and Mega electronvolt (MeV).
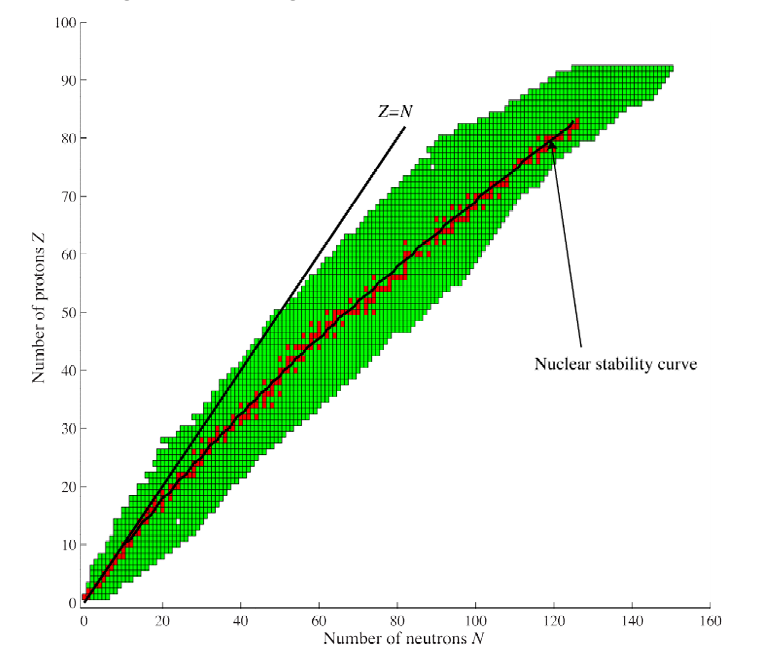
Compared to helium, which is a smaller atom, the larger atoms of higher atomic number elements will have larger mass defect and thus would require greater energy to break them apart. This doesn't mean that larger atoms are more stable ? Short answer is No. To understand the stability of atoms, we look at the bonding energy per nucleon (nucleon is a particle that makes up the nucleus and it can be a proton or a neutron). This is represented in the nuclear binding energy curve below.
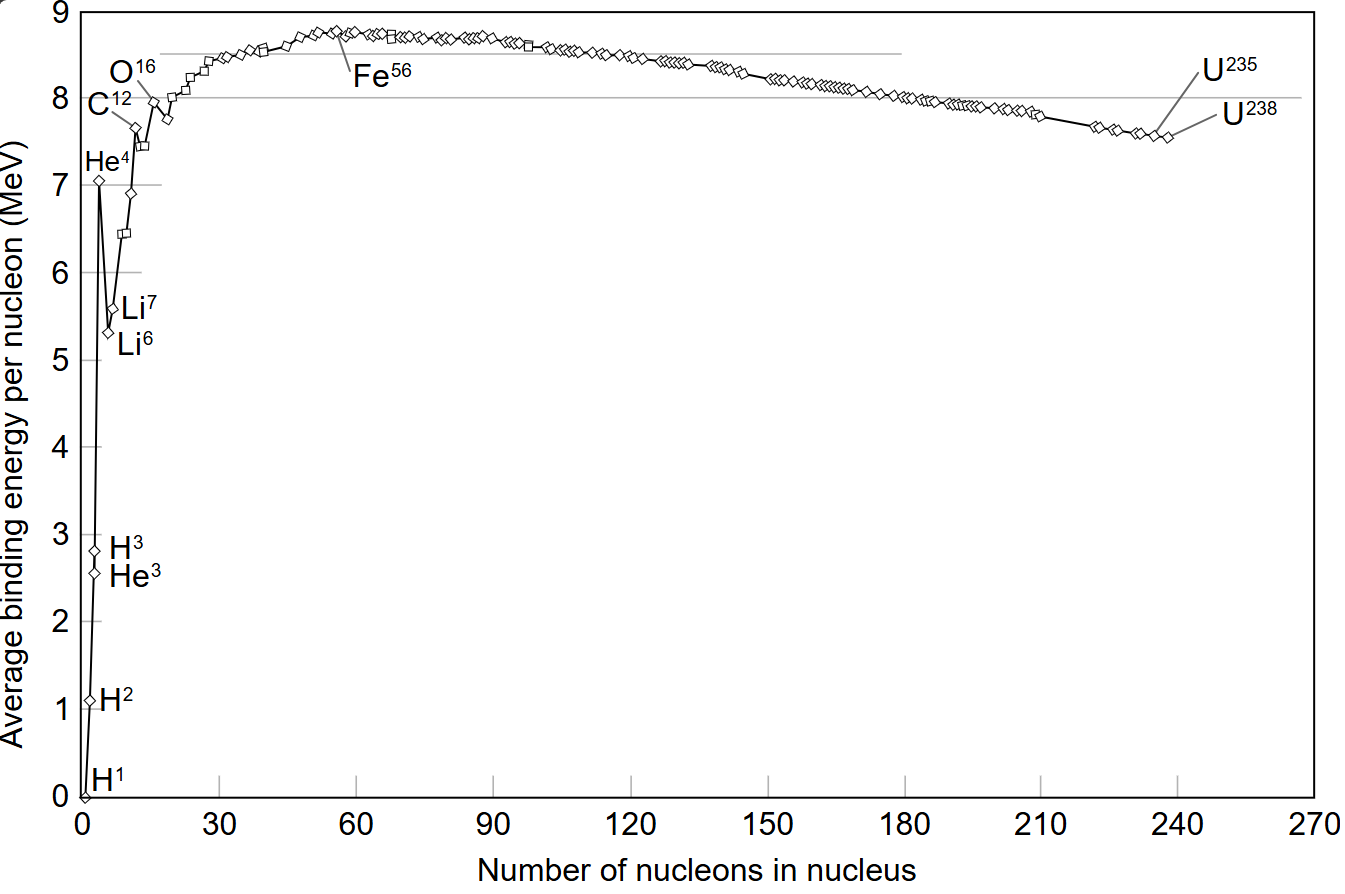
As you climb the chart of nuclides, every extra proton or neutron locks into the nucleus more tightly, raising the average binding energy per nucleon until the celebrated “iron peak” is reached. Iron‑56 boasts one of the strongest nucleon bonds in nature as the diameter of the nucleus of this is approximately equal to the range of strong nuclear force. Elements lighter than iron can liberate energy through fusion, while those heavier than iron yield energy when they split apart. This divide makes iron the pivotal “tipping point” in the periodic table’s energy balance.
At subatomic scales, the strong nuclear force binds protons and neutrons with immense strength, approximately 100 times the electromagnetic force at around one femtometer. But its influence wanes beyond about 2 - 3 fm, falling off exponentially with distance. In heavier nuclei, particularly those beyond iron, the long‑range Coulomb repulsion between positively charged protons begins to outpace the short‑reach of the strong force, eroding the stability of atoms and making fission more favourable.
Fission of nucleus like uranium or plutonium leads to comparitively lighter fission fragments with more stable nucleus compared to uranium and plutonium. Similarly, Fusion of hydrogen isotopes leads to formation of helium, which is comparatively more stable.
Any change that results in the movement of less stable nucleus to more stable nucleus results in the release of large amounts of energy. The more stable the nucleus, the more apparent the mass defect, this mass defect is converted into kinetic energy of fragments or photons in accordance with Einstein's mass-energy equivalence.
Fission fragments
Now coming back to nuclear fission, unlike what we have been told during our schooling, the fission fragments are not always the same. The nucleus, after absorbing a neutron, enters a highly excited state and its subsequent splitting is not a single deterministic event but rather a statistical process influenced by quantum mechanics and interplay of complex forces exerting at the nucleus.
Nuclear fission doesn’t split atoms into equal halves. Instead, fragments cluster asymmetrically into two mass groups (~90-100 AMU and ~130-140 AMU), shown by the bimodal mass-yield curve below. This occurs because splitting nuclei reorganizes protons and neutrons to maximize stability, prioritizing “magic numbers” and balancing the strong force with Coulomb repulsion. The process releases neutrons to shed excess energy and stabilize the neutron-rich fragments.
There can be hundreds of fission fragment isotopes depending upon the fission event. To streamline this complex data into usable probabilities, facilitating better planning and operational efficiency, we use fission yield, which presents a probabilistic distribution of products, reflecting the likelihood of each isotope's formation. Knowledge of fission yield is essential for reactor design, safety assessments and nuclear waste management.
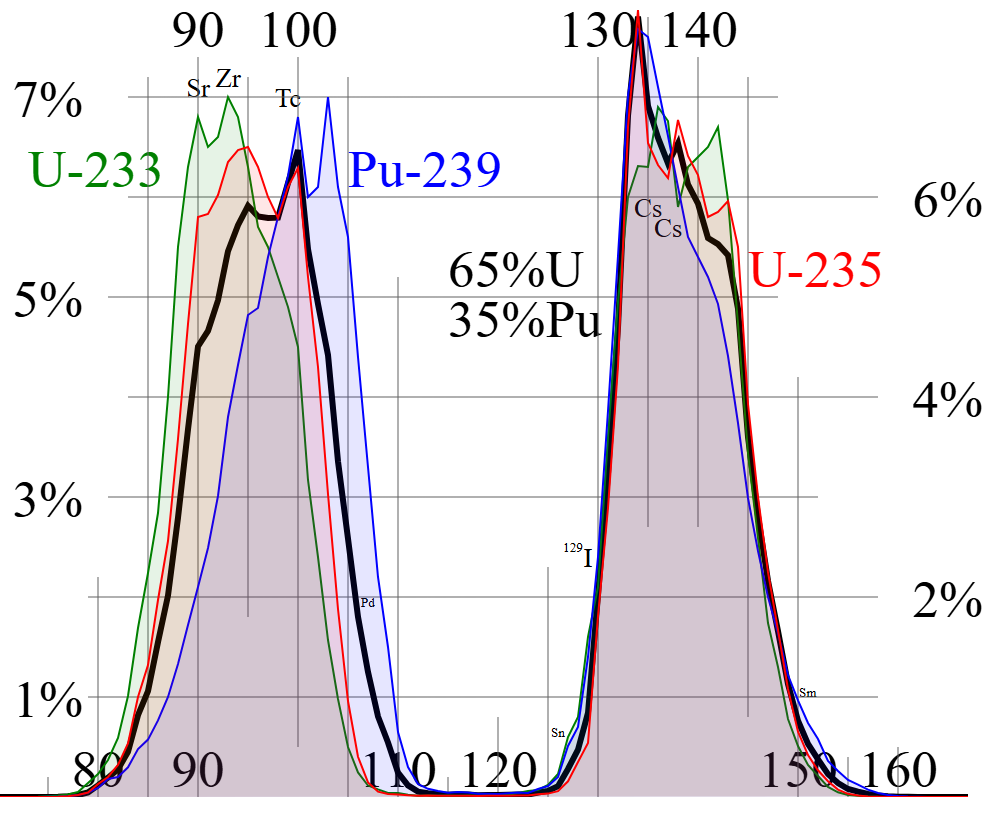
Uranium-235 and plutonium-239 are the backbone of nuclear energy because their fissile nuclei easily split when struck by neutrons, releasing massive energy, fission fragments and additional neutrons to sustain chain reactions. Their unique advantage lies in their ability to emit 2 - 3 neutrons per fission, enough to spark a self-sustaining chain reaction without requiring extremely high neutron concentrations. The number of neutrons released isn’t fixed, it depends on the specific isotope and conditions of the reaction. These neutrons collide with nearby fissile nuclei, triggering more splits and releasing even more energy and neutrons. Left unchecked, this domino effect escalates in a fraction of a second, producing immense power.
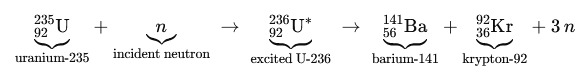
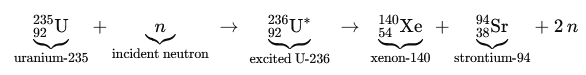
The two fission reactions we’ve explored, barium-krypton and xenon-strontium are textbook examples of uranium-235’s split. But here’s the twist: fission fragments aren’t confined to fixed isotopes. The same elements can emerge in different isotopic forms, subtly shifting the number of neutrons released. For instance, barium might appear as Ba-141, Ba-144, or other variants, tweaking the neutron tally. This isotopic flexibility explains why each fission event releases approximately 2.43 neutrons on average, a critical figure for sustaining chain reactions in reactors.
But the story doesn’t end there. The fission fragments can also pair up in entirely different elemental combinations. Below are some notable examples:
- Barium and Krypton
- Strontium and Xenon
- Cesium and Rubidium
- Iodine and Yttrium
- Zirconium and Lanthanum
- Cerium and Rubidium
Some of the fission fragment isotopes are inherently unstable, hence they further undergo radioactive decay to form more stable elements or isotopes. Unlike the lighter elements, as we go up in the atomic number, the stable nuclei require more neutrons than protons (N>Z) to counteract proton-proton electrostatic repulsion.
Electricity generation using nuclear fission
Building on our understanding of nuclear fission, let’s explore how reactors convert atomic splits into electricity, a process that hinges on heat transfer and mechanical energy conversion.
Here’s a simplified breakdown:
- Energy release from the nuclear fission.
- Kinetic Energy in the form of fast moving fission fragments hits the nuclear fuel, transfering the kinetic energy into the lattice structures causing strong vibrations, which increase the temperature.
- Gamma rays and delayed energy release from radioactive decay heat of fission fragments.
- Coolant circulates through the reactor serving as moderator and heat absorber.
- This coolant absorbs the thermal energy and transfers it away from the reactor core into a secondary water loop, where the water in the secondary loop convert into high pressure steam upon absorbing the thermal energy from the coolant.
- The cooled coolant goes back into the reactor to continue the process again, while the hot pressurized steam in the secondary loop is sent into the turbine chamber.
- This high pressure steam turns the turbine, which is coupled to a generator, at high speeds. The generator converts this rotational mechanical energy into electricity.
- The steam then slows down after transfering most of the kinetic energy into the turbine and this flows into a condenser to turn back into liquid. A part of this is merged with the cold water from the water storage that is sent into the secondary loop, while the other part of the water from the steam condensation is diverted into cooling towers or mixed with water in water storage.
Reactor technologies
Nuclear reactors are categorized into distinct types based on factors such as safety standards, environmental regulations, cost-effectiveness of cooling systems, fuel efficiency, regional infrastructure demands, technological innovation and the operational scale of power plants. We will provide a brief overview of the most widely deployed reactor technologies, listed in descending order of their global prevalence.
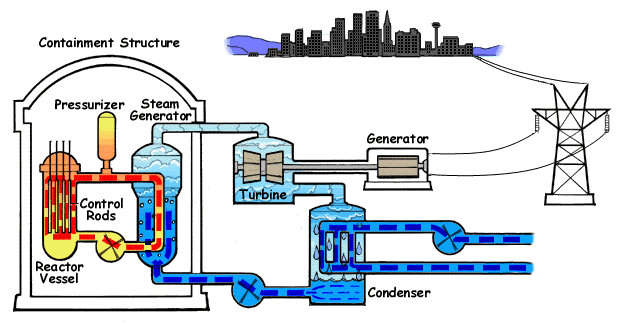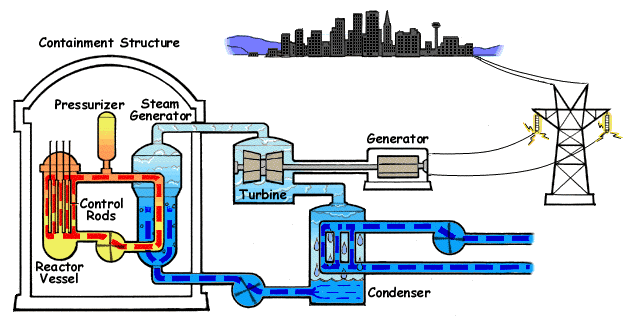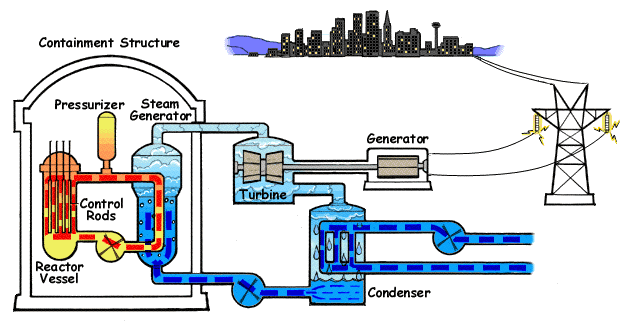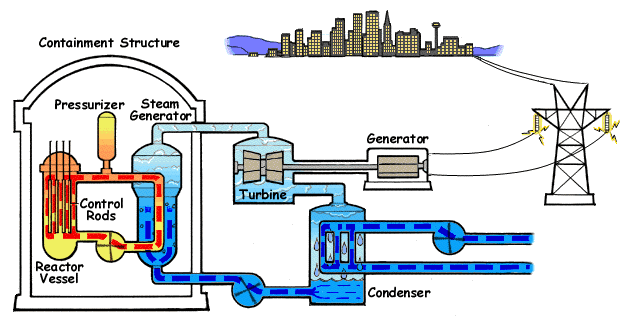
- Pressurized Water Reactor (PWR): A pressurized water reactor circulates high-pressure water through its core to absorb heat, which is then transferred via steam generators to a separate secondary loop where lower-pressure water boils into steam. This steam drives turbines to produce electricity, while the isolated primary loop ensures radioactive materials remain contained.
- Key Advantages:
- Safety: Separation of radioactive primary loop from non-radioactive secondary loop minimizes contamination risks.
- Efficiency: High-pressure operation allows efficient heat transfer.
- Key Advantages:
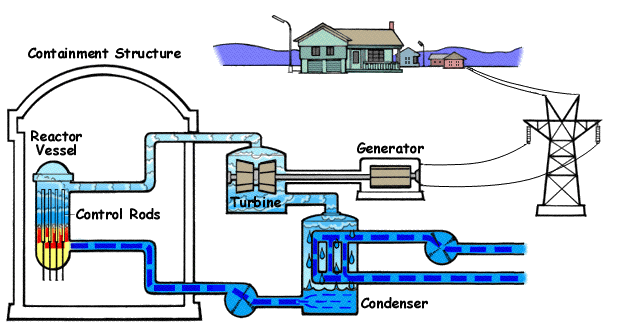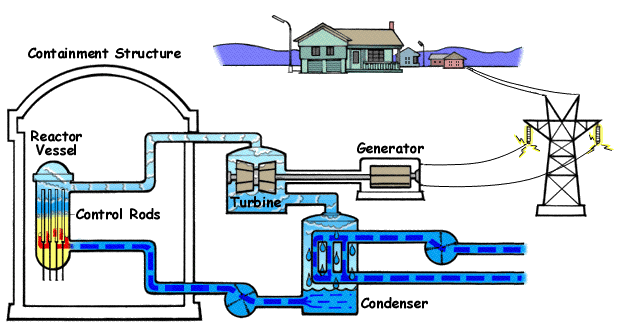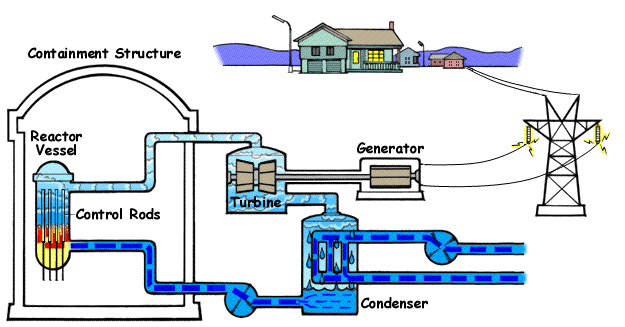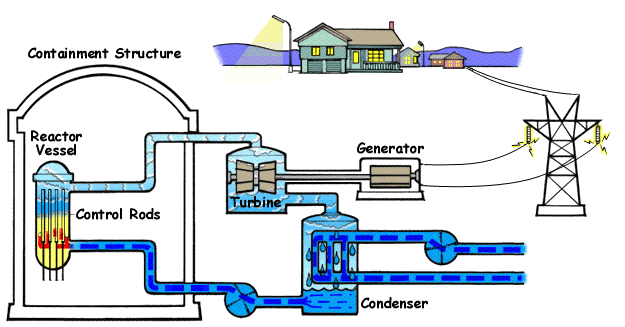
- Boiling Water Reactor (BWR): A boiling water reactor allows water to boil directly in its reactor core, producing steam that drives turbines to generate electricity. Unlike pressurized water reactors, these reactors operate with a single loop, eliminating the need for steam generators. The turbine and auxiliary equipment that are exposed to radioactive steam from the reactor core are housed in reinforced buildings with secondary containment systems to prevent environmental contamination.
- Key Advantages:
- Simplicity: Single-loop design reduces complex components like steam generators, lowering construction costs.
- Operational efficiency: The single-loop design eliminates steam generators, reducing complexity and energy loss. Operating at lower pressure than pressurized water reactors, direct steam production in the core simplifies energy conversion, enabling faster response to power demand changes.
- Key Advantages:
- Pressurized heavy water reactors: A Pressurized Heavy Water Reactor is a nuclear reactor design that uses heavy water (deuterium oxide, D₂O) as both the moderator and coolant, enabling the use of natural uranium (0.7% Uranium 235) as fuel. This eliminates the need for uranium enrichment, making these reactors cost-effective for countries with limited enrichment capabilities. These are distinct for their horizontal pressure tube design, allowing on-power refueling (fuel replacement without shutdown).
- Key Advantages:
- Natural uranium fuel: Natural uranium can be used directly, eliminating the need for domestic uranium enrichment plants or reliance on foreign enrichment services.
- On-power refueling: Pressurized heavy water reactors can refuel while operating, thanks to their horizontal pressure tube design. This minimizes downtime and maximizes energy output, as reactors do not need to shut down for fuel replacement
- Key Advantages:

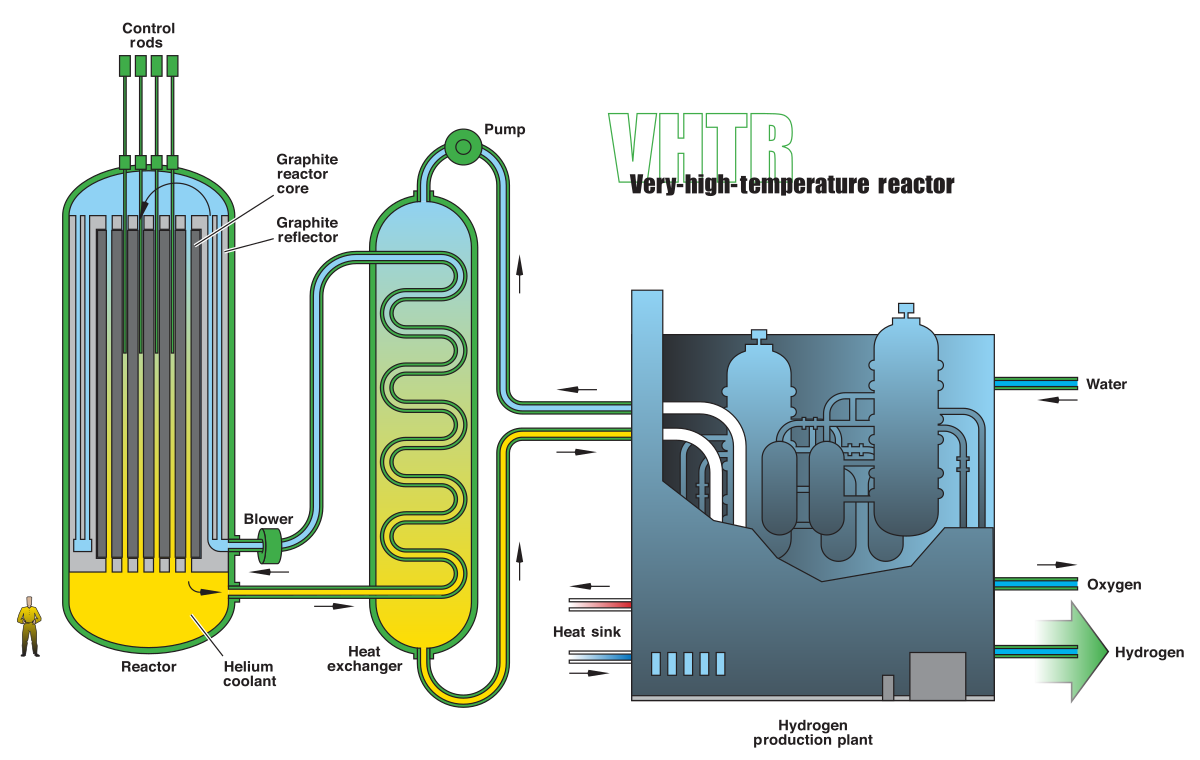
- Gas-cooled reactors (GCR/high temperature GCR/advanced gas reactor):
- GCRs use graphite as a neutron moderator and CO₂ or helium as a coolant, enabling natural uranium fuel operation without enrichment.
- High temperature GCRs employ helium coolant and graphite moderation to achieve temperatures up to 950°C, enhancing thermal efficiency (around 40%) and enabling hydrogen production, desalination processes and other industrial heat applications.
- Advanced gas reactors evolved from GCRs, using slightly enriched uranium oxide fuel and operating at higher temperatures (around 650°C) for improved efficiency.
- Key advantages:
- These avoid water-based cooling, ideal for arid regions. LWRs/HWRs require massive water volumes for cooling towers or seawater systems.
- TRISO (TRi-structural ISOtropic) fuel is a key innovation in advanced nuclear reactors, particularly high temperature GCRs. Its design inherently complicates plutonium extraction for weapons, making it a proliferation-resistant fuel.
- Key advantages:
- Small Modular Reactors (SMRs): Prefabricated nuclear power units optimized for factory production and modular on-site assembly. These are the scaled-down reactors with lower power production capacity. Their compact design enables scalable, low-carbon energy generation and deployment in diverse environments like isolated microgrids, industrial complexes and in regions with limited infrastructure.
- Key advantages:
- Lower costs and faster deployment: Reduce upfront capital investment and construction time through standardized factory production, cutting deployment timelines to 3–5 years.
- Deployment flexibility: Can be deployed in isolated regions or areas with constrained grid infrastructure, paired with renewable energy systems and adapted for non-power applications such as hydrogen generation or displacing coal facilities. Their modular scalability supports incremental energy capacity expansion as demand evolves.
- Key advantages:
Non-uranium 235 reactor technologies
While uranium 235 is the traditional focus in nuclear fission, two other fissile isotopes, uranium 233 and plutonium 239 also provide significant energy generation capabilities, each with distinct traits and applications. These materials require different production methods, exhibit unique nuclear properties and need specialized reactor designs for optimal use.
- Uranium 233 is produced through the thorium fuel cycle, a nuclear fuel cycle that employs thorium 232. Unlike natural uranium, thorium contains only trace amounts of fissile material insufficient to initiate a nuclear chain reaction independently. In the reactor, thorium 232 absorbs neutrons and transmutes into the fissile artificial uranium isotope, uranium 233, which serves as the nuclear fuel. (We’ll dive deeper into this topic in our upcoming article.. stay tuned.)
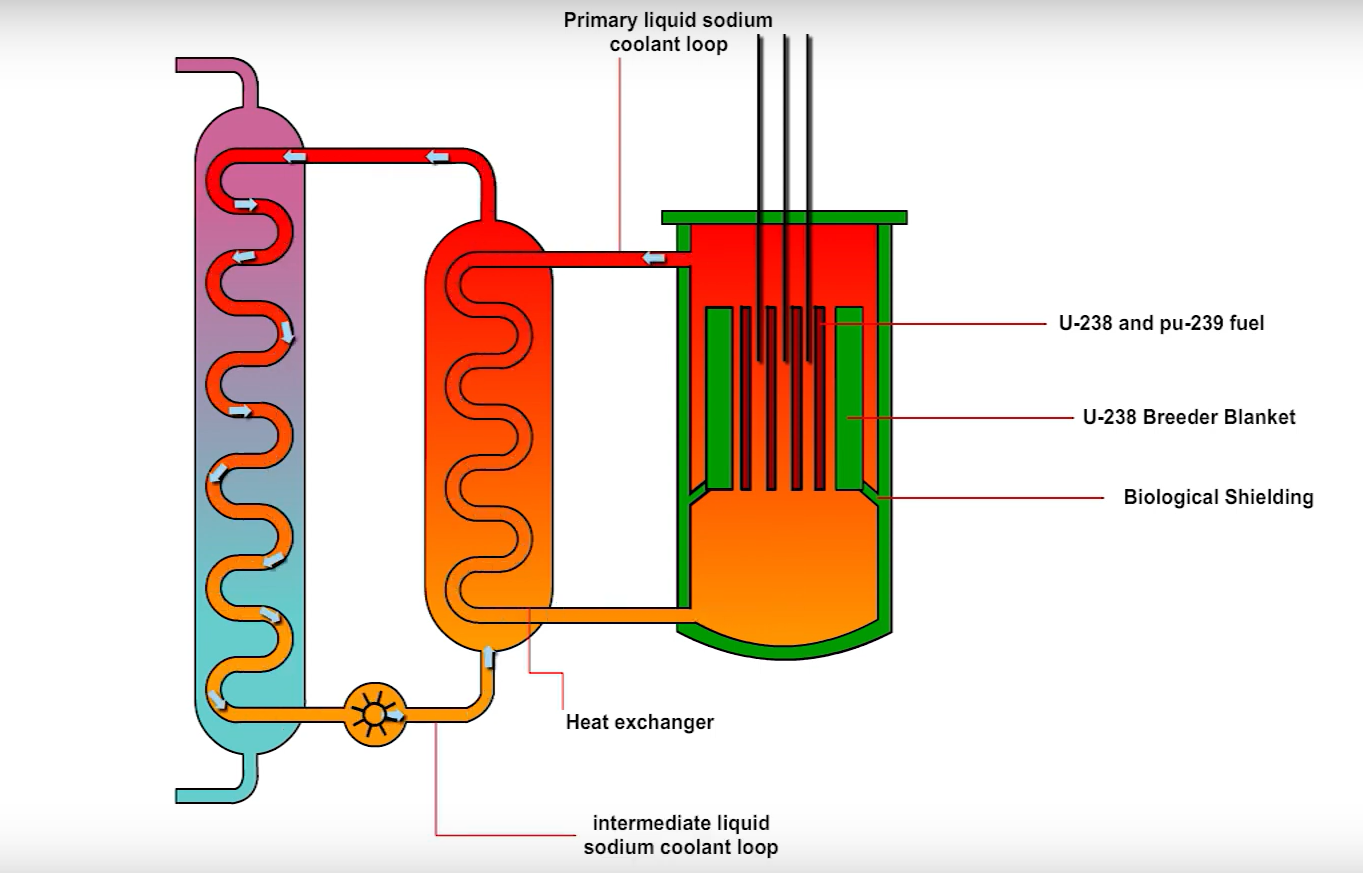
- Uranium 238, comprising over 99% of natural uranium, is non-fissile. Breeder reactors combine it with plutonium 239, which sustains fission with fast neutrons (unlike uranium 235). Molten sodium coolant preserves neutron speed, as it does not act as a moderator. In these reactors, uranium 238 absorbs neutrons, transmuting into uranium 239, then decaying to neptunium 239 and finally plutonium 239. Most fission products (like cesium 137, strontium 90) decay within centuries (half-lives approximately 30 years), unlike plutonium’s 24,000 year half-life. However, transuranic elements (like plutonium) persist far longer. Plutonium 239 faces limited adoption due to extreme toxicity (lethal if inhaled or ingested), complex processing costs and weapons-grade proliferation risks. Russia’s BN-600/BN-800 reactors repurpose Cold War-era plutonium stockpiles, originally intended for disarmament under the 2000 USA-Russia Plutonium Management Agreement. The USA abandoned its MOX fuel program and now dilutes plutonium with inert materials for underground storage. While reprocessing diluted plutonium is theoretically possible, it is difficult for weapons use. This agreement was mutually suspended in 2016 amid geopolitical disputes. These reactors remain operational, using plutonium-based fuel for electricity generation.
Amid the excitement about a self-sustaining fission chain reaction, we often overlook one crucial question: "how are the very first neutrons introduced?" The chain reaction doesn’t simply ignite on its own, well, it kind of does, via spontaneous fission, but the neutron flux from this process is insufficient for reliable startup. Operators instead use engineered startup sources, most commonly californium 252 or mixtures of alpha-emitters like americium-241 paired with beryllium, which release neutrons when alpha particles strike beryllium nuclei. At the same time, control rods are gradually withdrawn to carefully balance neutron loss against production until the chain reaction sustains itself.
Safety in nuclear fission
One of the primary considerations and a key skepticism against nuclear fission power plants is the safety aspect. There have been numerous nuclear disasters like the Fukushima and Chernobyl that shaped public sentiment on the nuclear power plants. Just as aviation transformed air travel by learning from decades of accidents, turning tragedies into fail-safe designs and protocols, nuclear energy has harnessed lessons from past failures.
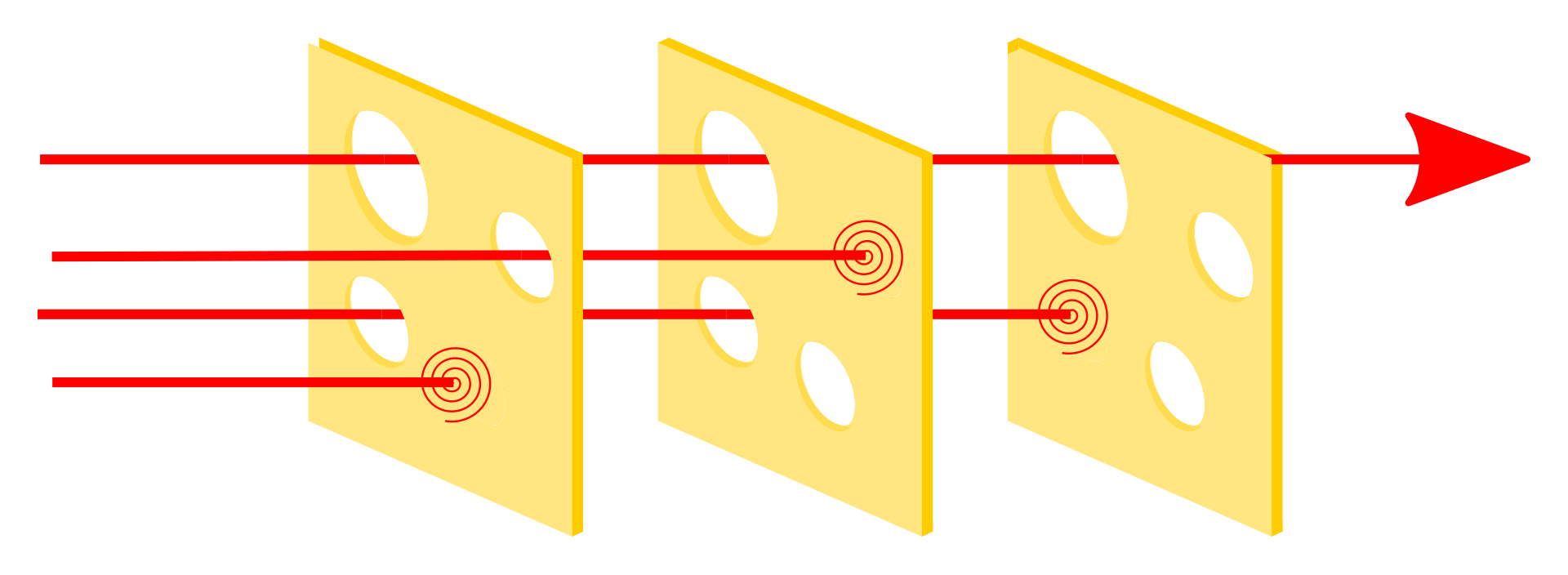
While no nuclear power plant can claim absolute safety, we implement a robust system of preventive measures engineered to operate collaboratively and autonomously. These layers of defense, each with built-in redundancies, ensure that no single failure can lead to catastrophic outcomes. The redundant safeguards act as backups, meaning a failure in one component is counterbalanced by others. For a significant safety breach to occur, multiple, unrelated failures would need to align simultaneously, a scenario visualized by the Swiss cheese model shown below. This model illustrates how overlapping layers of protection are structured to prevent risks from passing through all defenses at once, thereby maintaining safety even under unforeseen challenges.
Nuclear reactor safety demands three key criteria: controllable fission, passive cooling to manage post-shutdown decay heat and containment of radioactive materials. Let's analyse standard safety systems in reactors and their role in ensuring safe operations.
- Fuel cladding is a vital safety layer in nuclear reactors, acting as the primary barrier between radioactive fuel and the reactor environment. It contains fission-generated radioactive isotopes, prevents coolant-fuel chemical reactions, transfers heat to maintain safe temperatures and preserves structural integrity under extreme thermal, radiative and mechanical stresses. This ensures radioactive materials remain isolated while sustaining stable reactor operation
- Moderator (in case of uranium 235 based reactors coolant and moderator are both water) transfers heat from the reactor core to the secondary loop, which drives the turbine. Additionally, the moderator slows down fast moving neutrons, converting their kinetic energy into thermal energy, which the moderator/coolant removes. Slowing neutrons is critical: fast-neutrons are less likely to induce fission in uranium-235, as they pass by fuel nuclei too quickly to be captured. Thus, the chain reaction relies on moderated neutrons. If a coolant/moderator leak occurs, neutron speeds remain too high to sustain fission, inherently preventing an uncontrolled reaction.
- Control rods, composed of neutron-absorbing materials (like boron or cadmium), regulate fission by capturing excess neutrons to stabilize reaction rates and prevent runaway fission. During emergencies, the control rods automatically descend into the reactor core via gravity, rapidly absorbing neutrons to halt the chain reaction.
- Passive safety systems in nuclear reactors utilize natural forces, such as gravity, convection and phase changes, to maintain core cooling, remove decay heat and ensure containment integrity without operator intervention or external power. These systems regulate reactor pressure and temperature within safe limits. For instance, high-pressure borated water reservoirs automatically inject coolant during emergencies, suppressing reactions (negative reactivity) without pumps. Additionally, passive catalytic hydrogen recombiners prevent explosive hydrogen buildup (from zirconium cladding oxidation or radiation-induced water splitting) by safely combining hydrogen and oxygen into water vapor. This passive operation eliminates reliance on active components, enhancing safety during power outages or system failures. Triple-redundant cooling and backup systems ensure nuclear plant safety.
- The containment building, a steel-reinforced concrete radiological shield, serves as the final barrier preventing radioactive release during leaks or accidents. Engineered to withstand extreme steam/gas pressures from coolant malfunctions, it incorporates safety systems for steam/gas condensation. Some reactors utilize double-walled structures to enhance safety, redundancy and resilience against natural disasters or external threats.
Nuclear power remains one of the safest, most reliable energy sources available, yet public perception is still haunted by historical tragedies. From Hiroshima to Chernobyl and Fukushima, these events dominate conversations regarding the safety of nuclear energy. Both the Chernobyl and Fukushima disasters could have been preventable, or atleast wouldn't have turned into rank 7 disasters on the International Nuclear and Radiological Event Scale (INES).
- During a delayed nighttime safety test at Chernobyl, an inexperienced crew disabled critical protections and ran the reactor at an unstable low power level. A combination of design flaws, graphite-tipped control rods that briefly boosted reactivity and operator mistakes, including over-withdrawing rods and ignoring procedures, triggered an uncontrollable power spike and steam explosions that obliterated the core and unleashed massive radioactive contamination. This is the worst ever nuclear disaster in our history.
- The Fukushima nuclear disaster was directly caused by the magnitude 9.0 earthquake and subsequent tsunami, which flooded the plant, disabled backup power systems and led to reactor meltdowns due to the loss of cooling capabilities. However, the accident was exacerbated by preventable human and organizational failures, including inadequate tsunami preparedness, flawed safety regulations and poor emergency response planning by Tokyo Electric Power Company and Japanese authorities.
I wouldn't be able to fit in all the detials regarding both these nuclear disasters, I urge people to read articles or watch documentaries on these disasters.
Nobody died as a direct result of the Fukushima nuclear disaster. However, in 2018 one worker in charge of measuring radiation at the plant died of lung cancer caused by radiation exposure. In addition, there have been more than 2,000 disaster-related deaths. This classification includes deaths caused by suicide, stress and interruption of medical care. ~ extract from Britannica. However, a worker was reported to have died due to lung cancer caused by radiation exposure.
These nuclear catastrophes led to strict protocols and procedures, improved safety features and passive cooling systems, triple-redundant backup systems, improved and strict training for the control room crew and a whole lot more to make nuclear power generation safe for everyone.
In the nuclear power plants the fuel used is not enriched beyond 5%, unlike the nuclear bombs where the weapon-grade fuel enrichment can be beyond 90%. So, dont worry, the nuclear reactor cannot just explode like the nukes.
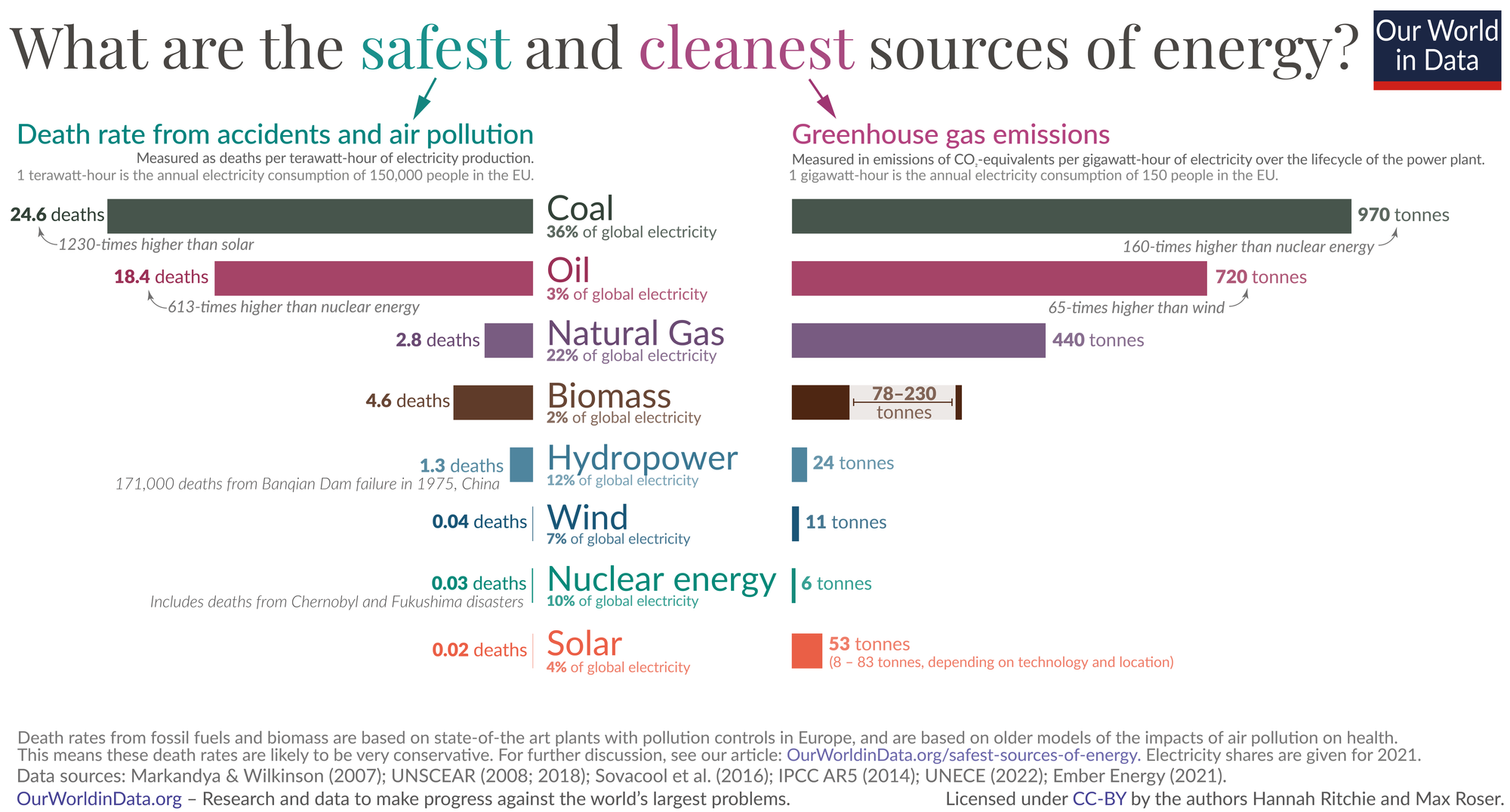
There have been significantly less number of deaths from nuclear related accidents compared to fossil fuel based energy generation, but any news regarding the nuclear power have always been linked to World War 2 bombings, Chernobyl and Fukushima disasters by the media and online articles. This shaped the public's negative perspective on the nuclear power plants. Its like saying airtravel is not safe and giving examples from the aircraft accidents through out history. Aviation industry has learnt from the accidents and made airtravel safer for everyone, this also holds true for nuclear power plants. That means operators and technical staff following rigorous protocols, regulators maintaining uncompromising oversight and engineers and builders refusing to cut corners in design or construction. When all stakeholders work together to enforce safety at every step, nuclear power can deliver its benefits with minimal risk.
Nuclear waste
One of the key arguments against nuclear power revolves around nuclear waste management. Nuclear waste, a subset of radioactive waste, is radioactive material generated from nuclear reactors, fuel processing, or weapons production, requiring specialized disposal. Depending upon the radioactivity, nuclear waste is categorized into 3 levels, low-level waste (LLW, includes the contaminated items like clothing and tools used), intermediate-level waste (ILW, such as the reactor components and chemical sludge) and high-level waste (HLW, like fission products and spent nuclear fuel).
- LLW contains minimal radioactivity and typically requires little to no shielding. After collection from facilities, it undergoes volume reduction via compaction, incineration, or encapsulation, depending on its activity. The processed waste is then securely placed in engineered near-surface disposal sites or specialized landfills designed with containment systems to prevent environmental exposure.
- ILW emits higher radiation, necessitating shielding and immobilization. It is stabilized within materials like cement, bitumen (a petroleum-based substance), or polymers to inhibit leakage, then sealed in radiation-blocking containers made of steel or concrete. These packages are stored in deep geological repositories or heavily reinforced near-surface vaults, isolated to ensure safety as the waste decays over hundreds of years to harmless levels.
- HLW primarily consists of spent nuclear fuel and waste generated from reprocessing of this used fuel. Spent nuclear fuel is intensely radioactive and generates significant heat, requiring robust shielding and cooling systems during handling and storage. These spent fuel rods are routinely submerged in water-filled storage pools for several years. Submerging spent nuclear fuel in water is a multifaceted safety strategy combining active cooling, radiation shielding, criticality control and environmental containment. This approach mitigates immediate risks while allowing time for radioactive decay, ensuring safe handling and eventual transfer to long-term storage solutions. HLW constitutes the lowest volume of overall nuclear waste of around 1%, yet accounts for over 95% of the total radioactivity.
In conventional nuclear reactors, not all of the fissile uranium is used up before the fuel rods had to be changed. Over time, the accumulation of neutron-absorbing fission products (like xenon 135) and the dominance of uranium 238 make it increasingly difficult for neutrons to sustain a stable chain reaction.
Fissile uranium 235 makes up only around 3% - 5% of the total uranium in the fuel rods, most of the remaining uranium is the uranium 238. When the fuel rod is removed out from the reactor, it still has more than 50% of the available fissile uranium 235. Overall, only around 5% of the total available uranium ( fissile U 235 that underwent fission + U 238 converted into plutonium) is utilized. This means that over 90% of the potential uranium fuel energy is still left in the fuel rods when it is removed from the reactor.
Many fission products created during nuclear fission are radioactive. Some isotopes like technetium 99 (half-life of 2,11,000 years) and iodine 129 (half-life of 15.7 million years) have extremely long half-lives, persisting for millennia or longer. However, the most radioactive fission products, like cesium 137 (half-life of 30 years) and strontium 90 (half-life of 29 years) decay much faster but pose significant short-term hazards. During reactor operation, neutrons are absorbed by uranium 238, initiating a process called neutron capture. This transforms U 238 into plutonium 239 and other transuranic elements (elements heavier than uranium) like americium and curium. These transuranics are also radioactive, with half-lives ranging from decades to hundreds of thousands of years.
By the end of a fuel rod’s life cycle, these isotopes accumulate and around 1% of the spent fuel’s mass consists of plutonium isotopes, primarily Pu 239 and Pu 240. While most plutonium undergoes fission during reactor operation to provide energy, a small fraction remains unburned and accumulates in the spent fuel. The radioactivity of these isotopes varies: short-lived isotopes emit intense radiation, while long-lived isotopes persist for millennia but with lower activity.
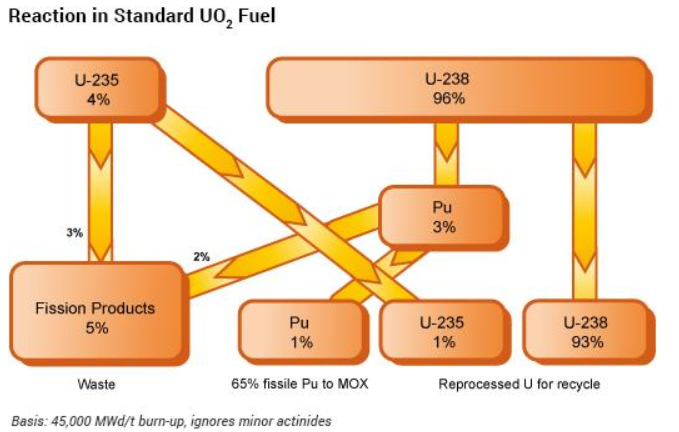
Spent nuclear fuel rods are initially cooled in storage pools before undergoing reprocessing. Government owned facilities or companies like Orano, a French leader in nuclear materials management, handle this complex procedure. The process involves mechanically separating the metal cladding from the radioactive fuel pellets. These pellets are subsequently dissolved in concentrated nitric acid, forming a liquid solution. A specialized solvent (commonly tributyl phosphate in industry-standard PUREX methods) is then introduced to chemically extract a mixture of uranium and plutonium, leaving behind fission products classified as high-level radioactive waste. To isolate plutonium from uranium, chemical additives induce redox reactions that alter plutonium’s oxidation state, enabling their separation through further solvent extraction.
The purified uranium solution undergoes additional solvent extraction and chemical treatments to remove residual fission products and organic solvents. It is then concentrated and converted into uranium oxide for reuse in nuclear fuel fabrication or interim storage. Recycled uranium can be reprocessed and fabricated into fuel assemblies for conventional nuclear reactors, reducing reliance on freshly mined uranium. Plutonium is purified through redox processes, precipitated as plutonium oxalate and calcined at high temperatures to form plutonium dioxide. This oxide is stored in helium-filled, airtight stainless steel containers to prevent oxidation, moisture absorption and criticality risks. The nitric acid-rich fission product solution is evaporated to concentrate radioactive isotopes, then blended with glass-forming additives. The mixture is heated in a vitrification melter (around 1,100°C) to create a homogeneous, chemically durable glass matrix. This molten glass is poured into stainless steel canisters, cooled and sealed. The vitrified waste is designed to immobilize radionuclides for hundreds of thousands of years and these canisters are deposited in deep underground storage facilities. Fuel rod cladding, end pieces and other metallic residues are decontaminated using acid rinses to remove residual fuel particles. These materials are categorized by radioactivity and material type, then compacted under high pressure into standardized packages. The compacted waste is encapsulated in steel containers for interim storage. Vitrified fission products and compacted metal waste are temporarily stored in engineered facilities to allow for heat dissipation and radiation decay. Long-term disposal in deep geological repositories is planned but not yet fully implemented globally. Core steps like solvent extraction and vitrification are standardized, but intricate procedures and storage protocols vary by country.
Nuclear waste processing reduces its volume to approximately one-fifth of the original, while enabling the recovery and reuse of uranium and plutonium for fabrication into mixed-oxide (MOX) fuel (consists primarily of a mixture of plutonium oxide and uranium oxide). MOX fuel demonstrates comparable operational efficiency to traditional uranium oxide fuel in reactors designed to accommodate its unique composition. The plutonium concentration is precisely adjusted to replicate the neutron economy of enriched uranium, ensuring seamless integration with reactor control and safety systems. Although MOX fuel exhibits slight variations in thermal conductivity and mechanical behavior relative to standard uranium fuel, these properties are well-characterized and mitigated through tailored engineering solutions, such as modified cladding designs or adjusted burnup rates during core management.
Spent MOX fuel is temporarily stored in water-filled cooling pools at reactor sites, mirroring the initial storage protocol for conventional spent nuclear fuel. Following years of cooling, it is often transferred to dry storage systems, such as passively cooled, helium-filled steel-concrete casks, for extended interim storage. Two long-term strategies are under consideration:
- Spent MOX fuel can be conditioned into corrosion-resistant copper or steel sealed canisters for permanent isolation in deep geological repositories.
- While technically feasible, reprocessing spent MOX fuel to recover plutonium and uranium is rarely implemented at scale. Key barriers include the accumulation of non-fissile plutonium isotopes which complicate reuse and the fact that advanced separation techniques remain experimental, technically complex and unproven in commercial settings.
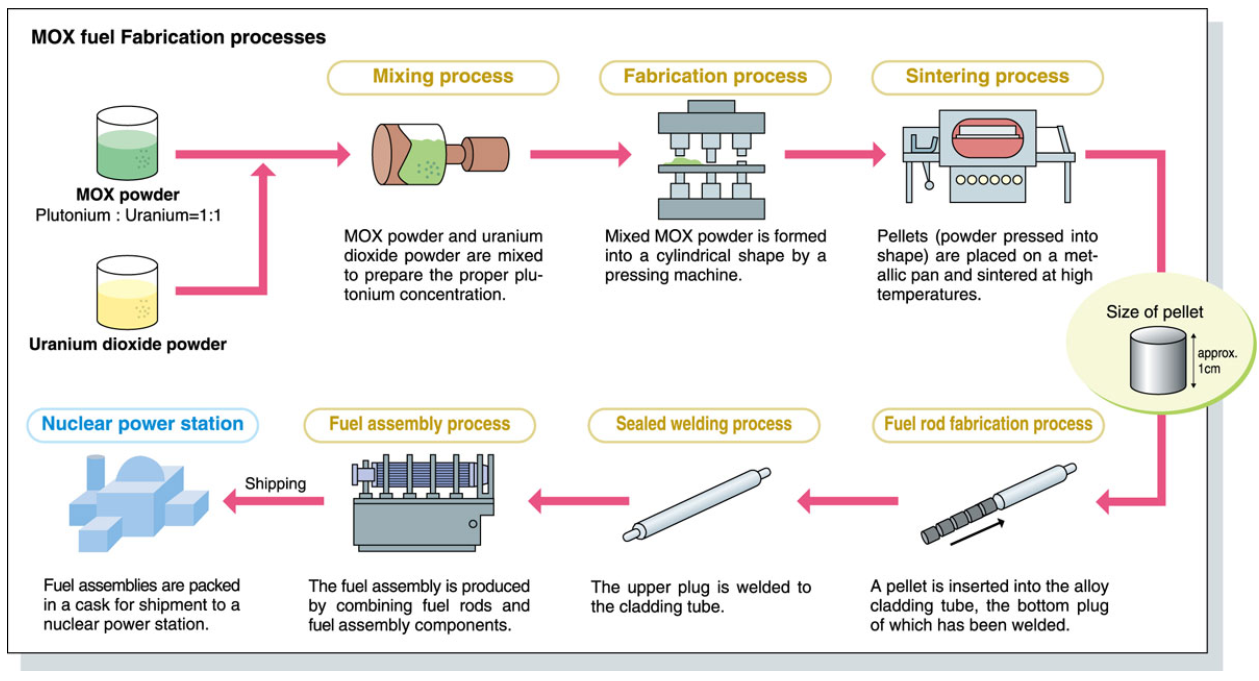
The reprocessing of spent nuclear fuel remains contentious due to its capacity to isolate weapons-grade plutonium, a key component in nuclear arms development. Concerns over proliferation risks, coupled with the high costs and technical complexity of waste treatment, have hindered the global adoption of fuel recycling programs. Fresh uranium retains a cost advantage, benefiting from mature mining infrastructure, streamlined supply chains and decades of industry optimization. However, advancing reprocessing technologies through targeted research and development could position recycled nuclear fuel as a financially competitive and sustainable alternative.
While international cooperation, collaborative R&D, and institutional safeguards play critical roles in curbing nuclear proliferation risks, a pragmatic supplementary approach involves leveraging multinational partnerships for spent fuel management. For instance, states could negotiate agreements with countries possessing advanced reprocessing capabilities, to convert spent fuel into proliferation-resistant forms like MOX fuel. Such arrangements would centralize expertise, minimize the global spread of sensitive technologies, and ensure recycled fuel is returned under strict safeguards for energy use, reducing both proliferation temptations and waste stockpiles.
Political dynamics hinder broader nuclear adoption, as governments initiating projects face heavy upfront costs with no immediate results; leaving them vulnerable to opposition criticism. By the time plants are operational (often after election cycles), the original proponents may no longer hold power to claim credit.
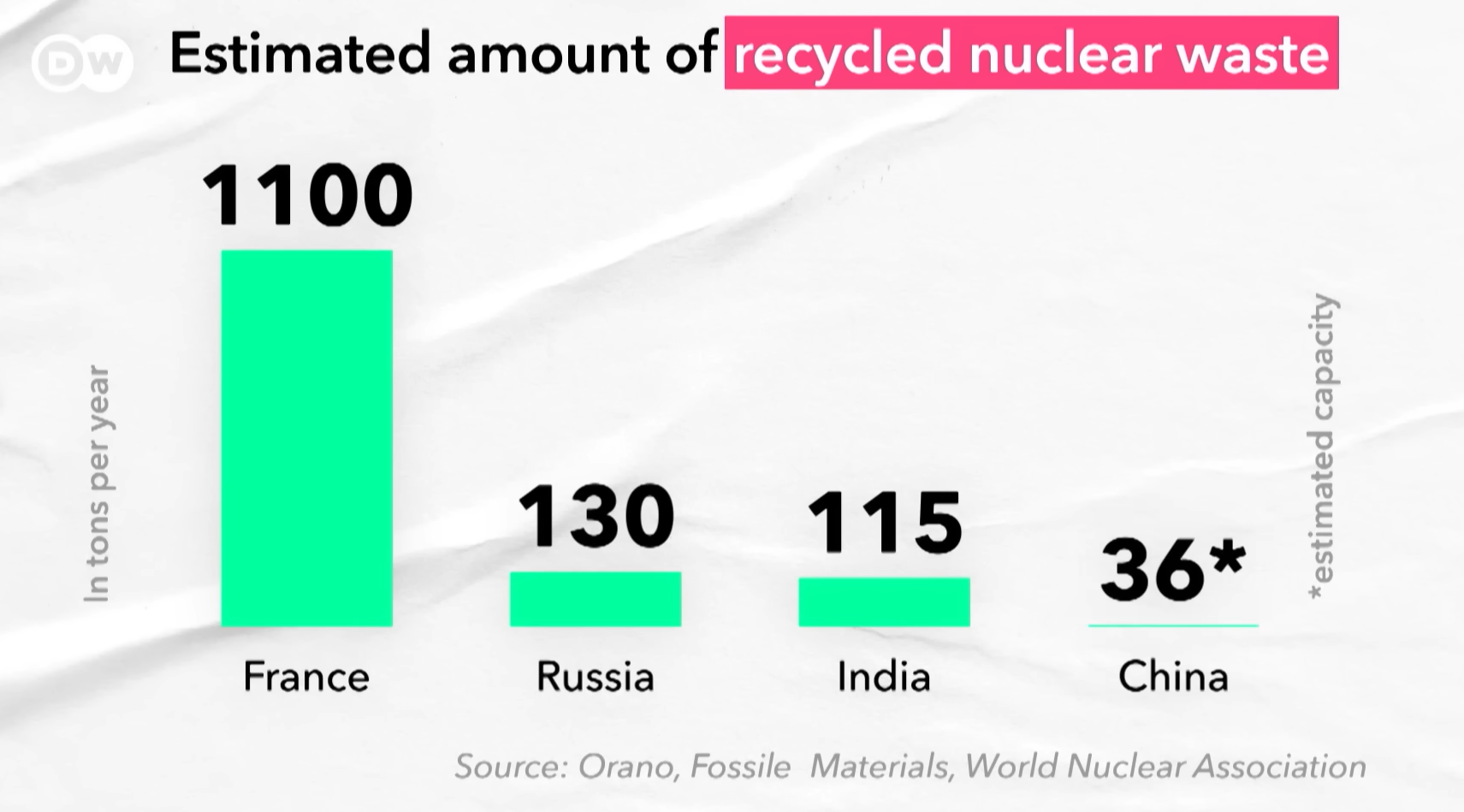
Complex economics of nuclear energy
Constructing nuclear power plants requires substantial upfront investment, primarily due to their large-scale infrastructure and the absence of standardized reactor designs in most countries. France serves as a notable exception, having implemented a centralized nuclear program with uniform reactor models. In contrast, many nations develop bespoke facilities tailored to site-specific conditions, regulatory frameworks and safety requirements. Each project undergoes rigorous design processes, repeated safety evaluations and compliance checks with international nuclear protocols to ensure operational reliability. These extensive procedures, driven by the need to mitigate risks and adhere to strict regulatory standards, result in prolonged development timelines and elevated capital expenditures compared to other energy infrastructure projects.
Nuclear fuels such as uranium or plutonium possess an energy density millions of times greater than that of fossil fuels per unit mass. Consequently, nuclear power plants require orders of magnitude less fuel to generate the same amount of electricity as conventional fossil fuel-based plants.
Lobbyists and industry interests in renewable and fossil fuel sectors often sway policy through advocacy, while nuclear power doesn't enjoy this advantage. The technical complexity and regulatory hurdles lead politicians to sideline or dismiss it as impractical.
The construction of nuclear power plants has historically faced challenges in adhering to timelines and budgets, though outcomes vary significantly based on project-specific factors. While some projects, such as South Korea’s Shin Kori-3 and -4 reactors, were completed near original deadlines and budgets, others like the Vogtle Plant in the USA or Finland’s Olkiluoto-3, experienced multi-year delays and cost overruns exceeding billions of dollars. These disparities highlight a mix of preventable and non-preventable causes, as well as the role of human judgment and systemic complexities.
Non-Preventable Challenges:
- External shocks: The COVID-19 pandemic disrupted global supply chains, labor availability, and material procurement, delaying critical components like reactor vessels. Similarly, geopolitical conflicts or trade restrictions can stall projects.
- Regulatory evolution: Changing safety standards mid-construction (like post-Fukushima requirements) often reflect improved safety practices but add delays and costs.
- Environmental and legal hurdles: Opposition from local communities or environmental groups, while sometimes rooted in misinformation, can lead to litigation or permit revisions that prolong timelines.
Preventable Challenges:
- Poor planning/design flaws: Underestimating project complexity such as incomplete reactor designs or inadequate site studies, leads to mid-construction revisions.
- Human error and expertise gaps: Inexperienced contractors, safety oversights (such as improper welding inspections),or rushed quality control can compromise progress.
- Financial mismanagement: Over-optimistic budgeting, opaque cost allocation, or reliance on untested technologies (like first-of-a-kind modular reactors) inflate expenses.
The economic viability of nuclear energy has long been a topic of significant discussion, with arguments both supporting and opposing it. To simplify the analysis, we’ll start with levelized cost of energy (LCOE). This metric calculates the average cost per unit of energy generated by a source over its operational lifespan, factoring in all associated expenses. By providing a standardized comparison, LCOE serves as a practical tool for evaluating diverse energy technologies, even those with differing capacities or operational scales.
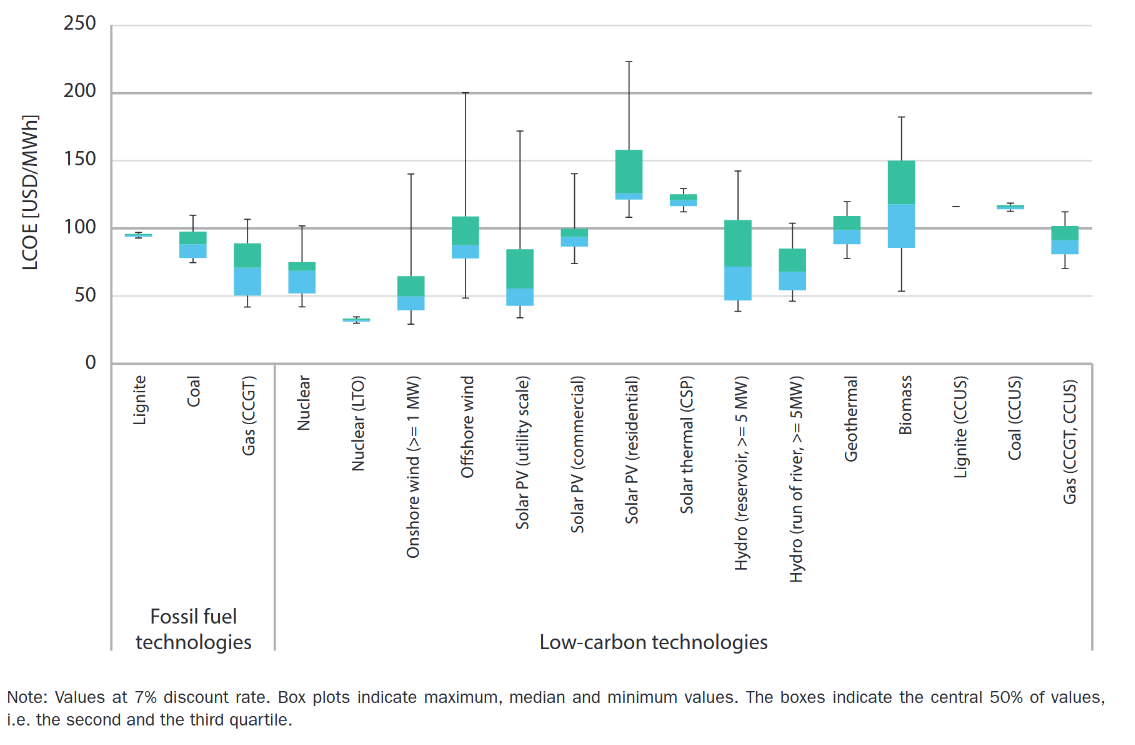
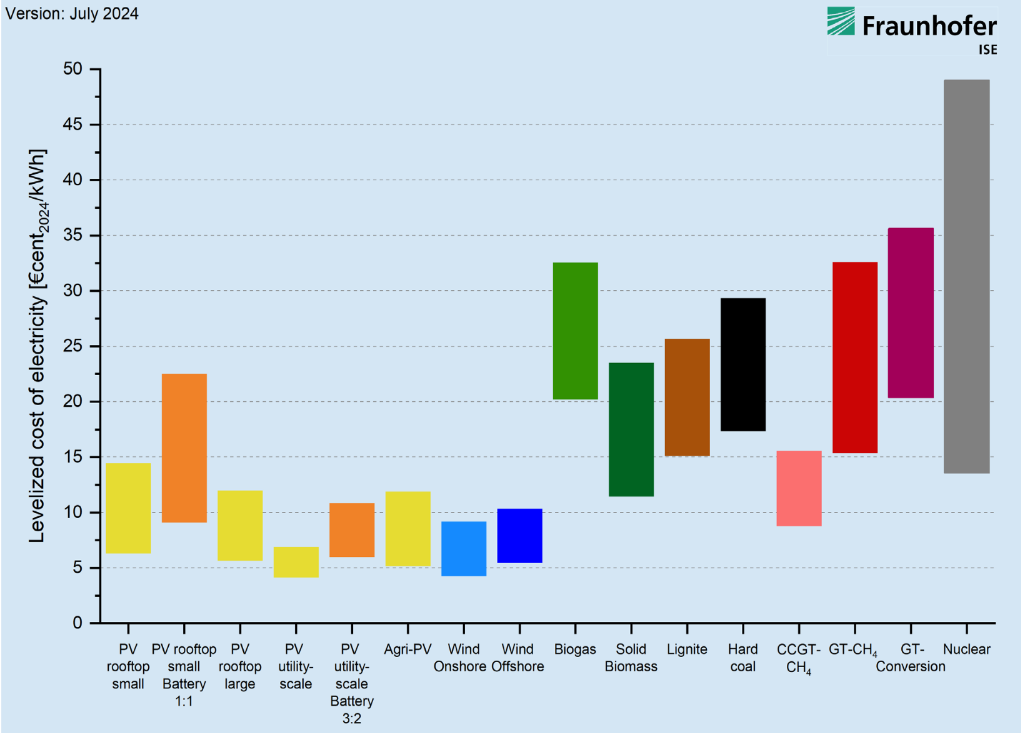
The above charts represent wildly different LCOE, depending upon where you see the charts from, the results can be inclining towards or away from nuclear. Let's look a bit deeper into the above LCOE comparision by Fraunhofer Institute for Solar Energy Systems ISE.
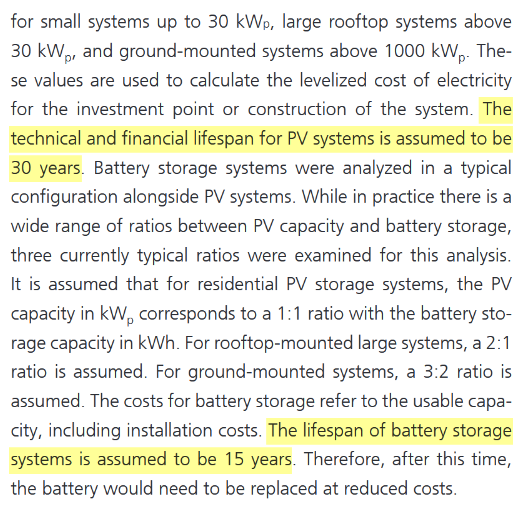
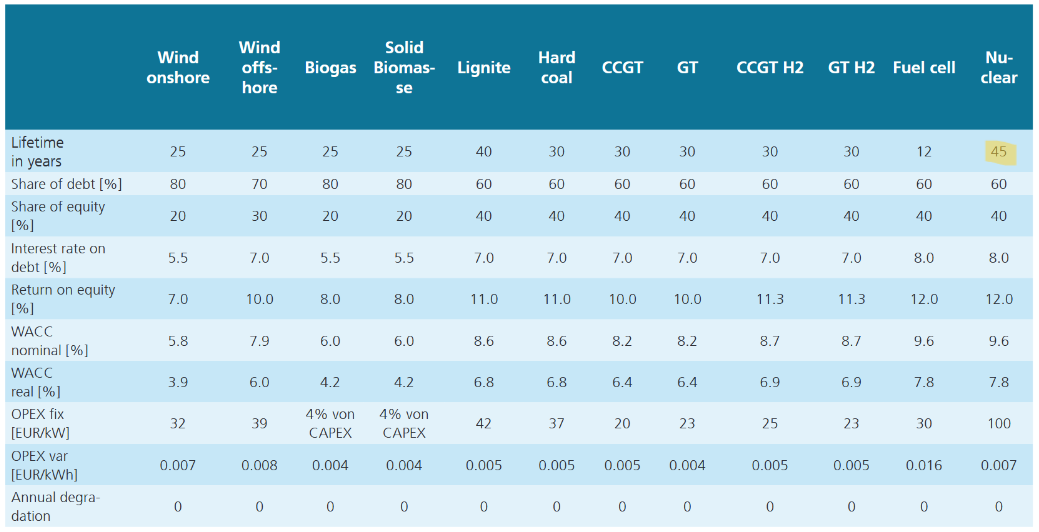
Let me address the primary concerns I’ve identified. Solar installations are projected to have a lifespan of 30 years, batteries approximately 15 years, and nuclear plants up to 45 years. However, these estimates require scrutiny. Photovoltaic systems experience gradual efficiency losses, and privately-owned solar power plants often replace all panels within 10–15 years to maintain output and meet rising energy demands. With annual energy demand projected to grow, the degradation of solar panels leads to an annual reduction in power generation, compounding the challenge. Roof-top solar may meet this 30 year lifespan. Even most of the state-owned solar power plants swap the panels by the 20 year mark. Regarding batteries, a 15-year lifespan appears overly optimistic. In practice, retaining functionality for 7–10 years without significant efficiency loss is exceptional. Modern nuclear plants are designed to operate for 60–80 years, with some facilities engineered to operate for up to a century. This starkly contrasts with the estimates applied to other energy sources.
Similarly, decomissioning of a nuclear power plant is not taken into consideration for LCOE that favours nuclear. Unlike other forms of energy production, nuclear power plants cannot be just aboandoned or directly reused. The decomissioning of a nuclear power plant is a significant expensive and technical undertaking. Moreover, the solar panels from a solar power plant can be reused or can be sold to hobbyists or home owners, yes the efficiency is reduced but still usable. This also reduces the overall expenditure.
As solar, wind and other renewables cannot produce power continuously, large‐scale energy storage solutions (like pumped hydro, batteries or gravity-based systems) is essential to buffer supply and ensure reliability. With electrification of almost every industry, data centres and AI driving ever-higher demand, retiring fossil generators without adequate storage risks grid instability and undermines energy security. Deploying renewables also requires careful siting—geology, land use, environmental impacts and transmission access constrain project locations.
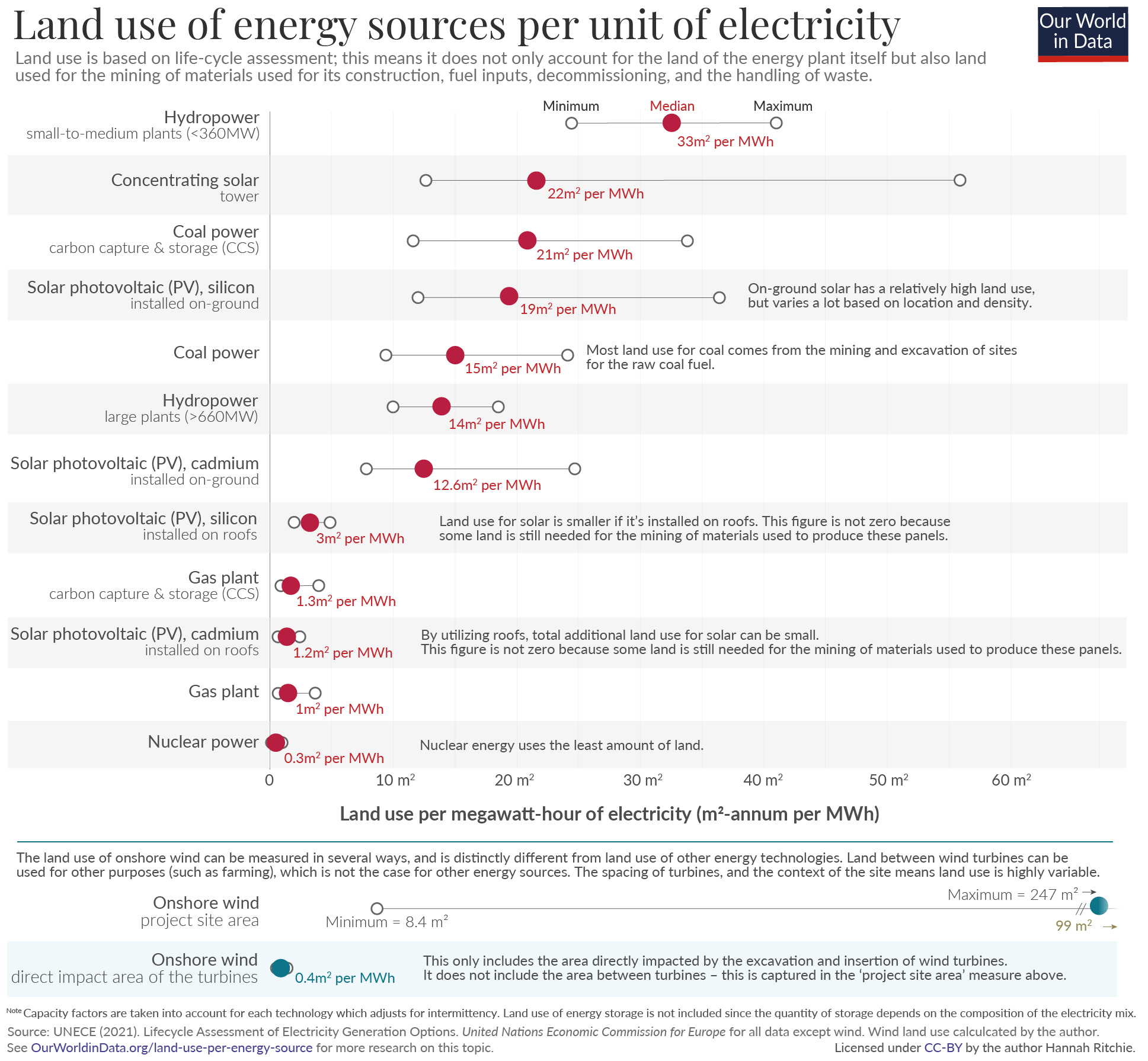
While nuclear power’s adjusted levelized cost of electricity (LCOE) aligns with the higher end of renewable energy costs, its advantages as a reliable, low-carbon energy source with a capacity factor exceeding 90% make it a compelling option. Even if nuclear energy incurs a higher cost per unit of electricity, it remains critical for ensuring energy security, diversifying the energy mix, and providing stable power generation in extreme weather or grid disruptions.
In contrast, overreliance on variable renewables like wind and solar necessitates significant investments in energy storage and grid infrastructure. These ancillary systems do not directly generate revenue; and their costs, whether borne through higher electricity prices or diverted public tax funds, ultimately fall on society. A balanced integration of nuclear and renewables, however, could optimize affordability, reduce dependency on external energy sources, and strengthen long-term resilience. This approach supports both decarbonization goals and energy independence without compromising grid reliability.
Nuclear energy is indispensable in the transition to a decarbonized future, offering a unique trifecta of 24 x 7 reliability, near-zero emissions, and unparalleled energy security. By providing a stable baseload power supply, it mitigates the intermittency challenges of renewables while reducing dependence on volatile fossil fuels or foreign energy imports. Paired with renewable expansion, nuclear strengthens grid resilience, ensures national energy independence, and accelerates the phaseout of carbon-intensive sources. A balanced, forward-looking energy strategy must leverage nuclear’s strengths to achieve both climate goals and a self-sufficient, resilient power system for generations to come.
For feedback or to report inaccuracies, kindly drop a mail at feedback@ecoethos.co
References





https://www.unene.ca/essentialcandu/pdf/3%20-%20Nuclear%20Processes%20and%20Neutron%20Physics.pdf












https://www.nrc.gov/docs/ML1210/ML12101A419.pdf
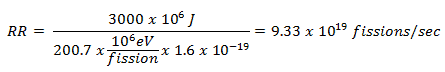


https://ukinventory.nda.gov.uk/wp-content/uploads/2014/01/Fact-sheet-spent-fuel-reprocessing.pdf
https://www.iaea.org/sites/default/files/bull602june20190.pdf
https://en.irsn.fr/sites/en/files/2023-09/IRSN_Report-Entreposage-2019-00903-EN.pdf